Description
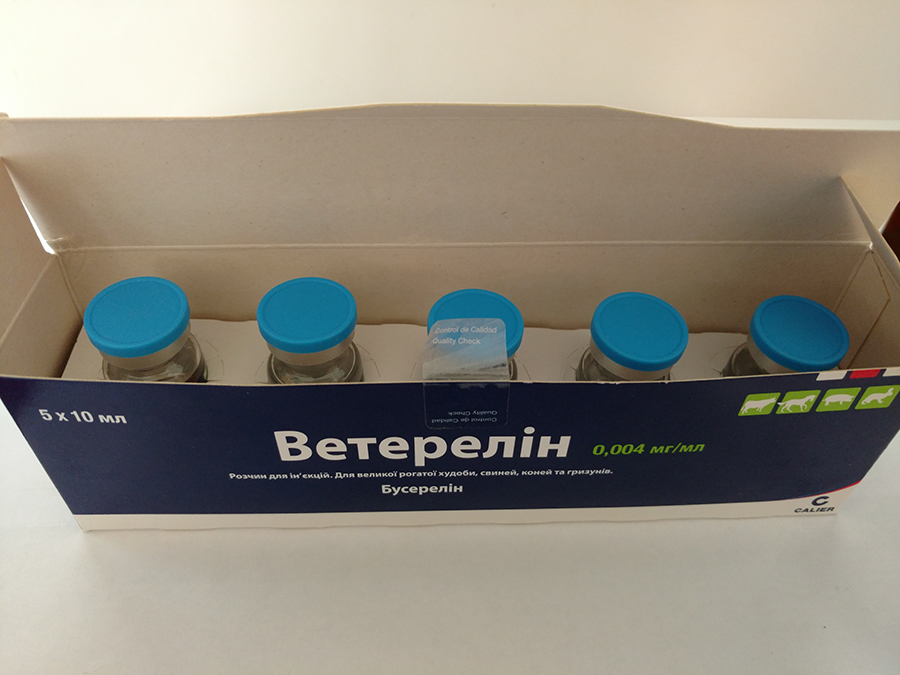
Buy Veterelin 0.004 mg/ml, solution for injection for cattle, horses, pigs, and rabbits
Description of Veterelin 0.004 mg:
Injectable solution for cattle, pigs, horses and rodents.
A ready-to-use colourless, aqueous injection solution for parenteral administration. Each ml contains 4 μg Buserelin and 10 mg benzyl alcohol, Ph.Eur., as antimicrobial preservative. Buserelin is equivalent to the natural LH/FSH releasing hormone produced in the hypothalamus. It causes simultaneous release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the anterior pituitary.
Packaging: Colourless glass vials of 10 (or 20) ml, with bromobutyl rubber closure and aluminium capsules with Flip-off opening ring in PP of blue colour.
The package contains 5 bottles of 10 ml.
Ingredients:
Active substance: Buserelin 0.004 mg (equivalent to 0.0042 mg Buserelin acetate);
Excipient: Benzyl alcohol 10 mg.
Pharmacologial Properties of Veterelin 0.004 mg/ml 10 ml:
For the treatment of infertility of ovarian origin and improvement of pregnancy rate in cows. For the synchronisation of oestrus in dairy cows and for reducing the calving to conception interval in these cows when used in conjunction with a PGF 2α analogue with luteolytic activity as part of a 10 day fixed time insemination regime. To induce ovulation of a mature follicle and thereby to synchronise ovulation more closely with mating in mares. To induce ovulation in pigs (gilts) after oestrus synchronisation in order to facilitate a single fixed time artificial insemination program. For the improvement of conception rate and induction of ovulation in rabbits. To facilitate stripping and reduce mortality due to egg binding in rainbow trout.
Pharmacodynamic Properties of Veterelin 0.004 mg/ml 10 ml:
Buserelin is a peptide hormone which is chemically analogous to the releasing hormone (RH) of the luteinising hormone (LH) and follicle stimulating hormone (FSH) thus a gonadotrophin releasing hormone (GnRH) analogue.
The mode of action of the veterinary medicinal product corresponds to the physiologic-endocrinological action of the naturally occurring gonadotrophin releasing hormone.
GnRH leaves the hypothalamus via the hypophyseal portal vessels and enters the anterior lobe of the hypophysis. Here it induces the secretion of the two gonadotrophins FSH and LH into the peripheral blood stream. These can act physiologically to cause maturation of ovarian follicles, ovulation and lutenization in the ovary.
Pharmacokinetic Properties of Veterelin 0.004 mg/ml 10 ml:
After intravenous administration, Buserelin is degraded rapidly: its half-life is 3 to 4.5 minutes in rat and 12 minutes in guinea pigs. Buserelin is accumulated in the pituitary gland, liver and kidneys where the substance is degraded by enzymes into small peptides fragments with negligible biologic activity. The main excretion route is in the urine.
Indications of Buserelin 0.004 mg 10ml:
FOR COWS (CATTLE)
FOR MARES (HORSES)
FOR RABBITS (DOES)
Improvement of conception rate and ovulation induction at post partum insemination.
FOR PIGS (Nulliparous cycling gilts )
Ovulation induction after oestrus synchronization with an analogue of progestagen (altrenogest) in order to perform a single artificial insemination.
Contraindications of Buserelin 0.004 mg 10ml:
Do not use in animals with known hypersensitivity to the active ingredient or to the excipients.
Precautions & Warning of Buserelin 0.004 mg 10ml:
Following withdrawal of the first dose, use the product within 24 hours. Discard unused material. Observe aseptic precautions.
Use of the product contrary to the recommended protocols may result in the formation of follicular cysts which may detrimentally affect fertility and prolificacy.
Avoid eye and skin contact with the solution for injection. In case of accidental contact, rinse thoroughly with water. Should skin contact with the product occur, wash the exposed area immediately with soap and water, as GnRH analogues may be absorbed through the skin.
Pregnant women should not administer the product, as buserelin has been shown to be foetotoxic in laboratory animals.
Women of child-bearing age should administer the product with caution.
When administering the product, care should be taken to avoid accidental self-injection by ensuring that animals are suitably restrained and the application needle is shielded until the moment of injection.
In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician. Wash hands after use.
Side effects:
None.
Interaction with other medicaments:
None known.
Overdose of Buserelin 0.004 mg 10ml:
In the case of repeated administrations of a dose corresponding to 3.5 ml of product, reduced food consumption may be observed in gilts after the 2-nd injection. This effect is transient and no specific treatment is required.
Withdrawal period:
Meat and offal: zero days;
Milk: zero hours.
Storage:
Store below 25°C. Protect from light.
Shelf life:
Shelf life of the veterinary medicinal product as packaged for sale: 24 months;
Shelf life after first opening the immediate packaging: 8 hours.




Reviews
There are no reviews yet.