Description
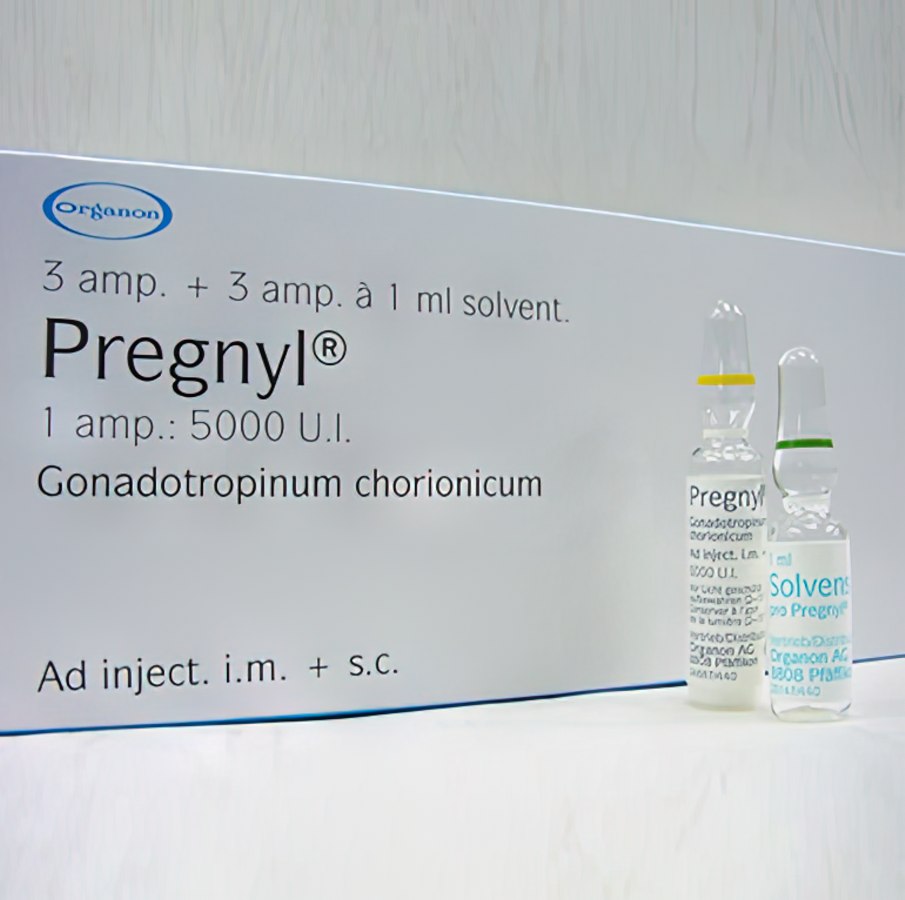
Buy Pregnyl HCG 5000 IU 1 ml of water solution
Description of Pregnyl HCG 5000 IU 1 ml of water solution:
Pregnyl (human chorionic gonadotropin (hcg)) is a glycoprotein that dissolves in water. It is available in the urine of pregnant women. It is composed of two sub-units namely; alpha and beta sub-units. A sole injection of hormone Pregnyl (human chorionic gonadotropin (hcg)) activates ovulation in a span of 38 to 40 hours. The action of Pregnyl (human chorionic gonadotropin (hcg)) is similar tothat of luteinizing hormone (lh). Along with the follicle stimulating hormone (fsh), lh influences the oogenesis process. Lh also initiates the ovulation process at mid-cycle. The role Pregnyl (human chorionic gonadotropin (hcg)) plays in the body is similar to that of lh that is, stimulation of ovulation in women having infertility problems. It triggers production of hormone progesterone by the corpus luteum in the ovaries.in men, it triggers secretion of hormone testosterone and also raises the sperm count. Pregnyl (human chorionic gonadotropin (hcg)) influencesthe interstitial cells in the testicles to produce androgens.
Dosage form
Powder for injection.
Basic physical and chemical properties: white dry powder or sticky mass;
The solvent is a clear and colorless aqueous solution.
Ingredients:
1 bottle (or 1 ml of ready-made solution) contains:
active substance: 1500 IU or 5000 IU of human chorionic gonadotropin;
other components: carmellose sodium; mannitol (E 421); sodium hydrogen phosphate, dihydrate; sodium dihydrogen phosphate, dihydrate.
1 vial with a solvent contains 1 ml of a 0.9% sodium chloride solution.
Pharmacodynamic Properties of Pregnyl HCG 5000 IU 1 ml of water solution:
Pregnyl contains human chorionic gonadotropin (hCG) with luteinizing hormone (LH) activity. LH is necessary for the growth and maturation of gametes in males and females, as well as for the production of sex steroid hormones.
For women, Pregnyl should be used as a mid-cycle replacement for endogenous LH surge to induce the final phase of follicular maturation, leading to ovulation. Pregnyl can also be used as a substitute for endogenous LH during the luteal phase.
Pregnyl should be used to stimulate Leydig cells to accelerate the formation of testosterone.
Pharmacokinetics Properties of Pregnyl HCG 5000 IU 1 ml of water solution:
Absorption and distribution. Peak plasma levels of lCH following a single intramuscular (IM) or subcutaneous (S/W) injection will be reached after about 6 and 16 hours, respectively, in men and women, in both cases, after about 20 hours. Although high inter-subject variability was observed, the sex-related difference after IM injection may be due to the greater thickness of adipose tissue in the gluteal region in women than in men.
Biotransformation. LHG is metabolized by about 80%, mainly in the kidneys.
Conclusion. The IM and PO routes of lCH administration have been found to be bioequivalent in terms of absorption volume and apparent half-life, which is approximately 33 hours. Given the recommended dosage regimens and the length of the half-life, cumulation is not expected.
Indications for use of Pregnyl HCG 5000 IU 1 ml of water solution:
Women:
- stimulation of ovulation in case of infertility caused by anovulation or impaired maturation of follicles;
- preparation of follicles for puncture in programs of controlled ovarian stimulation (in medical programs of assisted reproduction);
- support of the luteal phase in women during controlled ovarian hyperstimulation (in medical assisted reproduction programs) using gonadotropin-releasing hormone analogs or other means to stimulate ovulation in female infertility caused by anovulation in the absence of endogenous group activity.
Men:
- hypogonadotropic hypogonadism;
- infertility associated with idiopathic dyspermia.
Boys:
- delayed puberty associated with insufficient production of gonadotropin by the pituitary gland;
- cryptorchidism not associated with anatomical obstruction.
Contraindications of Pregnyl HCG 5000 IU 1 ml of water solution:
Women and men.
Hypersensitivity to human gonadotropins or to any of the components of the drug.
Diagnosed or suspected sex hormone-dependent tumors of the ovaries, mammary glands, uterus, testicles, prostate, pituitary gland or hypothalamus.
Extras for women.
Congenital pathology of the reproductive organs, in which pregnancy is contraindicated.
Fibroid tumor of the uterus, in which pregnancy is contraindicated.
Pathological (non-menstrual) vaginal bleeding without established/diagnosed etiology.
Side effects of Pregnyl HCG 5000 IU 1 ml of water solution:
Intramuscular or subcutaneous administration of the drug Pregnyl can lead to local reactions at the injection site. Most local reactions were mild and temporary. Rarely, generalized hypersensitivity reactions have been observed. Unwanted ovarian hyperstimulation syndrome is a potentially serious adverse reaction of Pregnyl.
Adverse drug reactions are listed below by body/organ system class and frequency. The frequency is defined as: very often (>1/10), often (>1/100, <1/10); infrequently (> 1/1000, <1/100); rarely (>1/10000, <1/1000); very rare (< 1/10000) and not known (cannot be estimated from available data).
From the side of the immune system
Rare: generalized hypersensitivity reactions (eg, generalized rash or fever).
Violations of the general condition and associated with the way the drug is used
Rarely allergic reactions at the injection site.
Not known: local reactions at the injection site (bruising, pain, redness, swelling and itching).
Women.
From the vascular system
Rare: thromboembolism associated with the treatment of FSH / lCH, usually in severe OHSS.
From the respiratory tract, chest and mediastinum
Not known: hydrothorax as a sign of severe COS.
From the gastrointestinal tract
Common: Abdominal and gastric disturbances (such as nausea and diarrhea) as a sign of mild OHSS.
Not known: ascites as a complication of severe COS.
From the reproductive system and mammary glands
Unwanted ovarian hyperstimulation, mild (often) and severe (rare) form of ovarian hyperstimulation syndrome.
Mild OHSS: breast tenderness, ovarian enlargement (mild to moderate), ovarian cyst, abdominal pain, abdominal discomfort, gastrointestinal symptoms (such as nausea, diarrhea, and flatulence).
Severe OHSS: large ovarian cysts (prone to rupture), acute abdominal pain, ascites, weight gain, hydrothorax, rarely thromboembolic complications due to the use of FSH / lCH.
Not all symptoms described are always associated with COS.
Survey
Not known: weight gain as a sign of severe COS.
Men.
From the side of metabolism and nutrition
Infrequently, water and sodium retention in the body was observed after the use of the drug in high doses (it is considered the result of excessive production of androgens).
From the reproductive system and mammary glands
Rare: Treatment with hCG may lead to gynecomastia.
Interaction with other medicinal products:
The interaction of the drug Pregnyl with other drugs has not been studied; therefore, interactions with commonly used drugs cannot be ruled out.
After administration, Pregnyl may interfere with the immunological determination of plasma/urine lCH for up to 10 days, and a pregnancy test may be pseudo-positive.
Overdose:
The acute toxicity of urine-derived gonadotropin preparations has been shown to be very low. However, there is a possibility that a very high dose of lCH can cause ovarian hyperstimulation syndrome.
Warning:
Women and men:
Hypersensitivity reactions:
Hypersensitivity reactions, both general and local, anaphylaxis and angioedema have been reported. If a hypersensitivity reaction is suspected, Pregnyl should be discontinued and other potential causes of the reaction evaluated.
General features:
- Patients should be evaluated for uncontrolled non-gonadal endocrinopathy (eg, thyroid, adrenal, or pituitary dysfunction) and specific treatment should be initiated.
- The active ingredient in this medicine is derived from human urine, so the risk of transmission of a pathogen (known or unknown) cannot be ruled out. There are no cases of viral contamination due to the use of gonadotropins derived from human urine.
- Patients on a sodium-restricted diet. This medicinal product contains less than 1 mmol sodium (23 mg) per daily dose, i.e. practically no sodium.
- Pregnyl should not be used to reduce body weight. LCH does not affect fat metabolism, fat distribution, or appetite.
Women.
Multiple pregnancy and childbirth:
Pregnancy after ovulation induction with gonadotropic drugs has an increased risk of multiple pregnancies.
Ectopic pregnancy:
Women suffering from infertility and using assisted reproductive technologies (ART) have an increased incidence of ectopic pregnancy. Early confirmation of intrauterine pregnancy by ultrasound is important.
Loss of pregnancy:
The frequency of pregnancy loss in women undergoing ART is higher than in the healthy population.
Congenital pathology:
The incidence of congenital malformations of the fetus after the use of Assisted Reproductive Technologies, as well as in patients with anovulation, may be slightly higher than after spontaneous conception. This is thought to be due to differences in parental characteristics (eg, maternal age, semen characteristics) and a higher rate of multiple pregnancies. There is no indication that the use of gonadotropins is associated with an increased risk of congenital malformations.
Ovarian hyperstimulation syndrome (OHSS):
OHSS is a medical condition that is distinct from uncomplicated ovarian enlargement. Clinical signs and symptoms of mild to moderate OHSS include abdominal pain, nausea, diarrhea, mild to moderate ovarian enlargement, and ovarian cysts. Severe OHSS can be a life-threatening condition. Clinical signs and symptoms of severe OHSS: large ovarian cyst, acute abdominal pain, ascites, pleural effusion, hydrothorax, dyspnea, oliguria, abnormal blood counts, and weight gain. In rare cases, venous and arterial thromboembolism may develop as a result of OHSS. SCOS has also been reported to be associated with transient pathological changes in liver function tests, with or without morphological changes on liver biopsy.
It is necessary to observe the recommended dose of the drug Pregnil and the treatment regimen. Caution should be exercised when prescribing Pregnyl, as OHSS can be caused by taking human chorionic gonadotropin (hCG). OHSS can also be due to pregnancy (endogenous hCG). Early OHSS usually occurs within 10 days of hCG use and may be associated with an excessive ovarian response to gonadotropin stimulation. Late OHSS occurs more than 10 days after the use of hCG, due to hormonal changes during pregnancy. Since there is a risk of developing OHSS, patients should be monitored for at least 2 weeks after the use of hCG.
Women with known risk factors for high intensity ovarian response may be particularly susceptible to developing OHSS during or after treatment with Pregnyl. Women with partially known risk factors receiving their first cycle of ovarian stimulation are advised to be closely monitored for early signs and symptoms of OHSS.
It is necessary to use modern clinical experience to reduce the risk of OHSS when using Assisted Reproductive Technologies (ART). Careful monitoring of ovarian response is essential to reduce the risk of OHSS. Ultrasonography should be performed for treatment and at regular intervals during treatment to monitor the development of CSOS; simultaneous determination of serum estradiol levels may also be appropriate. With ART, there is an increased risk of developing OHSS with 18 or more follicles that are 11 mm or larger in diameter. For patients at increased risk of OHSS or developing OHSS, standard and appropriate treatment for OHSS should be provided.
Torsion of the ovary:
Ovarian torsion has been reported following treatment with gonadotropins, including Pregnyl. Ovarian perversion may be associated with other conditions such as OHSS, pregnancy, previous abdominal surgery, a history of ovarian perversion, and a history or current ovarian cyst. Damage to the ovary due to reduced blood flow can be prevented if a timely diagnosis is made and the perversion is immediately corrected.
Vascular Complications:
Thromboembolic complications, either related or not related to OHSS, have been reported following treatment with gonadotropins, including Pregnyl. Intravascular thrombosis of both venous and arterial vessels can lead to reduced blood flow to vital organs or limbs. Women with recognized risk factors for thrombosis, such as a personal or family history of thrombosis, obesity, or thrombophilia, are at an increased risk of venous or arterial thromboembolic events during or after treatment with gonadotropins. In these women, the benefits of in vitro fertilization (IVF) treatment and possible risks should be assessed. It should be noted that pregnancy itself increases the risk of thrombosis.
Men.
Antibody formation:
The use of lCH can provoke the formation of antibodies against lCH. In rare cases, this can lead to treatment failure.
Treatment with hCG leads to an increase in androgen production. That’s why:
Patients with latent or severe heart failure, impaired renal function, arterial hypertension, epilepsy or migraine (or with a history of such diseases) should be under constant medical supervision, since an increase in androgen production can sometimes cause deterioration or recurrence of the disease.
Boys.
LCH should be used with caution in prepubertal children to avoid premature closure of the epiphyseal growth plate or precocious puberty. The indicators of the development of the skeletal system should be checked regularly.
Use during pregnancy or lactation.
Fertility.
The drug Pregnyl promotes fertility.
The drug Pregnyl is used to treat women who are undergoing ovulation induction or controlled ovarian stimulation in controlled ovarian stimulation programs. For men, the drug is used to treat a deficiency in spermatogenesis caused by hypogonadotropic hypogonadism.
Pregnancy.
Pregnyl is not indicated for use during pregnancy. There are no data on the use of human chorionic gonadotropin during pregnancy. Animal studies have demonstrated reproductive toxicity from the use of lCH. The possible risk to humans is unknown. Pregnyl can be used during pregnancy to maintain the luteal phase and should not be used later in pregnancy.
Lactation.
No information is available from clinical and animal studies regarding the passage of lCH into milk. It is unlikely that lCH passes into human breast milk because of its high molecular weight. Even if lCH were to pass into breast milk, it would be broken down in the baby’s gastrointestinal tract.
The ability to influence the reaction rate when driving vehicles or operating other mechanisms.
The drug Pregnyl does not affect the ability to drive vehicles or work with other mechanisms.
Storage:
Store in the original packaging at a temperature of 2 to 8 °C. Don’t freeze. Keep out of the reach of children.
Shelf life:
3 years.
Package:
Pregnyl®, powder for injection, 5000 IU: 3 bottle with powder and 3 bottle with 1 ml of solvent (0.9% sodium chloride solution) in a cardboard box.




Reviews
There are no reviews yet.