Description
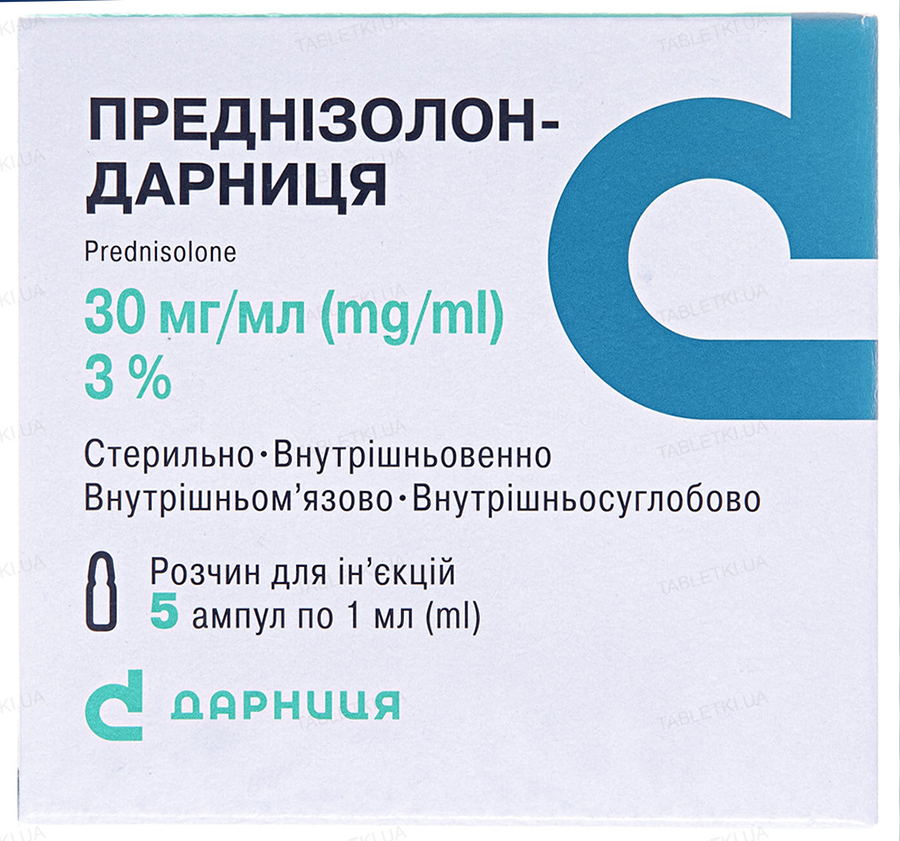
Buy Prednisolone 30 mg/ml 1 ml #5
Description of Prednisolone 30 mg/ml:
Injection.
Basic physical and chemical properties: transparent colorless or slightly colored liquid.
Pharmacological group: Corticosteroids for systemic use. Glucocorticoids.
This form Prednisolone is given as an injection, usually only done in a hospital. It can also be given is available as tablets and as a liquid to drink.
Ingredients of Prednisolone 30 mg/ml:
Active substance:
Prednisolone; 1 ml of solution contains prednisolone sodium phosphate in terms of prednisolone 30 mg;
Excipients:
Disodium edetate, disodium phosphate dodecahydrate, potassium dihydrogen phosphate, ethanol 96%, propylene glycol, water for injection.
Pharmacologial Properties of Prednisolone 30 mg/ml:
Pharmacodynamic:
- It has anti-inflammatory, anti-allergic, immunosuppressive, anti-shock and anti-toxic effects. In relatively large doses, it inhibits the activity of fibroblasts, the synthesis of collagen, reticuloendothelium and connective tissue (inhibition of the proliferative phase of inflammation), delays synthesis and accelerates protein catabolism in muscle tissue, but increases its synthesis in the liver. The antiallergic and immunosuppressive properties of the drug are due to the inhibition of the development of lymphoid tissue with its involution during long-term use, a decrease in the number of circulating T- and B-lymphocytes, inhibition of mast cell degranulation by inhibition of antibody production;
- The anti-shock effect of the drug is due to an increase in the vascular response to endo- and exogenous vasoconstrictor substances, with the restoration of the sensitivity of vascular receptors to catecholamines and an increase in their hypertensive effect, as well as a delay in the excretion of sodium and water from the body;
- The antitoxic effect of the drug is associated with stimulation of protein synthesis processes in the liver and acceleration of the inactivation of endogenous toxic metabolites and xenobiotics in it, as well as with an increase in the stability of cell membranes, including hepatocytes;
- It enhances the deposition of glycogen in the liver and the synthesis of glucose from the products of protein metabolism. An increase in blood glucose levels activates the secretion of insulin. Suppresses the uptake of glucose by fat cells, which leads to the activation of lipolysis. However, due to an increase in insulin secretion, lipogenesis is stimulated, which contributes to the accumulation of fat. Reduces calcium absorption in the intestines, increases its leaching from bones and excretion by the kidneys. It suppresses the release of adrenocorticotropic hormone and β-lipotropin by the pituitary gland, and therefore, with prolonged use, the drug may contribute to the development of functional insufficiency of the adrenal cortex;
- The main factors limiting long-term therapy with prednisone are osteoporosis and Itsenko-Cushing’s syndrome. Prednisolone inhibits the secretion of thyroid-stimulating and follicle-stimulating hormones;
- In high doses, it can increase the excitability of brain tissue and help lower the seizure threshold;
- Stimulates excess secretion of hydrochloric acid and pepsin in the stomach, and therefore may contribute to the development of peptic ulcers.
Pharmacokinetic:
- When administered intramuscularly, it is rapidly absorbed into the blood, but compared to reaching the maximum level in the blood, the pharmacological effect of the drug is significantly delayed and develops in 2-8 hours. In blood plasma, most prednisolone binds to transcortin (cortisol-binding globulin), and when the process is saturated, to albumin. With a decrease in protein synthesis, a decrease in the binding capacity of albumins is observed, which can lead to an increase in the free fraction of prednisolone and, as a result, the manifestation of its toxic effect when using conventional therapeutic doses. The half-life in adults is 2-4 hours, in children it is shorter. Biotransformed by oxidation mainly in the liver, as well as in the kidneys, small intestine, bronchi. Oxidized forms are glucuronized or sulfated and excreted in the form of conjugates by the kidneys;
- About 20% of prednisolone is excreted from the body by the kidneys unchanged, a small part is excreted in the bile;
- In liver diseases, the metabolism of prednisolone slows down and the degree of its binding to blood plasma proteins decreases, which leads to an increase in the half-life of the drug.
Indication of Prednisolone 30 mg/ml:
Intramuscular, intravenous administration:
- systemic connective tissue diseases: systemic lupus erythematosus, dermatomyositis, scleroderma, periarthritis nodosa, ankylosing spondylitis;
- hematological diseases: acute hemolytic anemia, lymphogranulomatosis, granulocytopenia, thrombocytopenic purpura, agranulocytosis, various forms of leukemia;
- skin diseases: common eczema, erythema multiforme exudative, pemphigus vulgaris, erythroderma, exfoliative dermatitis, seborrheic dermatitis, psoriasis, alopecia, adrenogenital syndrome;
- replacement therapy: Addison’s crisis;
- emergency conditions: severe forms of ulcerative colitis and Crohn’s disease, shock (burn, traumatic, surgical, anaphylactic, toxic, transfusion), status asthmaticus, acute adrenal insufficiency, hepatic coma, severe allergic and anaphylactic reactions, hypoglycemic conditions.
Intra-articular administration:
Chronic polyarthritis, osteoarthritis of large joints, rheumatoid arthritis, post-traumatic arthritis, arthrosis.
Contraindications of Prednisolone 30 mg/ml:
- Hypersensitivity to the components of the drug;
- Parasitic and infectious diseases of a viral, fungal or bacterial nature that currently exist or have recently been transferred: herpes simplex, herpes zoster (viremic phase), chicken pox, measles; amoebiasis, strongyloidiasis (established or suspected); systemic mycosis; active tuberculosis;
- Post-vaccination period (duration 10 weeks: 8 weeks before and 2 weeks after vaccination), lymphadenitis after BCG vaccination.
Immunodeficiency states caused by HIV infection; - Diseases of the gastrointestinal tract: peptic ulcer of the stomach and duodenum, esophagitis, gastritis, acute or latent peptic ulcer, recent intestinal anastomosis, ulcerative colitis with the threat of perforation or abscess formation, diverticulitis;
- Diseases of the cardiovascular system: recent myocardial infarction, decompensated chronic heart failure, arterial hypertension, tendency to thromboembolic disease;
- Diseases of the endocrine system: decompensated diabetes mellitus, thyrotoxicosis, hypothyroidism, Itsenko-Cushing’s disease;
- Severe chronic renal and / or liver failure (with the exception of such an emergency as hepatic coma), nephrourolithiasis;
- Hypoalbuminemia. Systemic osteoporosis. Myasthenia gravis. severe myopathy. Productive symptoms in mental illness, psychosis. Obesity (III-IV degree). Poliomyelitis (with the exception of the form of bulbar encephalitis). Open and closed angle glaucoma, cataract;
- For intra-articular, periarticular and tendon sheath injections where there is infection at the site of injection or in surrounding tissues;
- For injection directly into the tendon;
- Injections into spinal or other non-diarthrodial joints.
Interaction with other medicinal products:
Co-treatment with CYP3A inhibitors, including products containing cobicistat, is expected to increase the risk of systemic side effects. This combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side effects, in which case close monitoring of the patient is necessary.
Possible reactions with the simultaneous use of prednisolone with other drugs:
- with thyroid hormones, inducers of liver enzymes, in particular with barbiturates, phenytoin, pyrimidone, carbamazepine, rifampicin – a weakening of the effects of prednisolone as a result of an increase in its systemic clearance;
- with rifampicin, rifabutin, carbamazepine, phenobarbital, phenytoin, primidone, ephedrine and aminoglutethimide – a decrease in the therapeutic effect due to an increase in the metabolism of corticosteroids;
- with estrogens (including oral contraceptives containing estrogen), cyclosporine, CYP3A4 inhibitors, in particular erythromycin, clarithromycin, ketoconazole, diltiazem, aprepitant, itraconazole, oleandomycin – increased therapeutic and toxic effects of prednisolone, so dose adjustment may be necessary;
- with etoposide – inhibition of metabolism by glucocorticosteroids in vitro is possible. This may lead to an increase in both the efficacy and toxicity of etoposide, so monitoring is necessary;
- with antacids – reduced absorption of prednisolone;
- with derivatives of salicylic acid and other non-steroidal anti-inflammatory drugs – an increase in the likelihood of bleeding and ulcers of the gastric mucosa. Prednisolone reduces the level of salicylic acid derivatives in the blood serum, increasing their renal clearance, and the excretion of steroids can lead to salicylate intoxication. The drug increases the risk of developing hepatotoxic reactions of paracetamol as a result of the induction of liver enzymes and the formation of its toxic metabolite. Aspirin should be used cautiously in combination with glucocorticosteroids in patients with hypoprothrombinemia;
- with coumarin anticoagulants and warfarin – their effectiveness can be enhanced by simultaneous therapy with corticosteroids. Therefore, careful monitoring of the international normalized ratio (INR) or prothrombin time is necessary to avoid spontaneous bleeding;
- with cardiac glycosides – increased toxicity of the latter, and as a result of the resulting hypokalemia – an increased risk of developing arrhythmias;
- with hypoglycemic agents – suppression of the hypoglycemic effect of oral hypoglycemic agents and insulin;
with antihypertensive drugs – a decrease in the effectiveness of the latter; - with tricyclic antidepressants – increased signs of depression caused by taking prednisolone, and increased intraocular pressure;
- with immunosuppressants – an increased risk of developing infections and lymphoma or other lymphoproliferative disorders associated with the Epstein-Barr virus;
- with diuretics (loop, thiazide and acetazolamide), carbenoxolone, laxatives and antifungals (amphotericin B) – an increased risk of hypokalemia, so concomitant use should be avoided. There is also an increased risk of hypokalemia with concomitant use of theophylline and with high doses of corticosteroids concomitantly with high doses of bambuterol, fenoterol, formoterol, ritodrine, salbutamol, salmeterol and terbutaline. Prednisolone increases the risk of developing osteoporosis when used simultaneously with amphotericin and carbonic anhydrase inhibitors;
- with ketoconazole – the metabolic and renal clearance of methylprednisolone is reduced, which is also possible with prednisolone;
- with mifepristone – it is possible to reduce the effect of corticosteroids within 3-4 days;
- with methotrexate – an increased risk of developing hematological toxicity is possible;
- with m-anticholinergics, antihistamines, nitrates – an increase in intraocular pressure and a decrease in the effectiveness of antihistamines;
- with antipsychotics, carbutamide, azathioprine – an increased risk of developing cataracts;
- with estrogens, anabolic drugs, oral contraceptives – manifestations of hirsutism and acne. It is possible to increase the effect of glucocorticoids, therefore, in this case, dose adjustment is necessary;
- with live antiviral vaccines and against the background of other types of immunizations – an increase in the risk of activation of viruses and the development of infections. High doses of corticosteroids worsen the immune response, so the use of live vaccines should be avoided;
- with muscle relaxants against the background of hypokalemia – increased severity and duration of muscle blockade against the background of the use of muscle relaxants;
- with anticholinesterase agents – the occurrence of muscle weakness in patients with myasthenia gravis (especially in patients with myasthenia gravis) and a decrease in the effect on cholecystographic x-ray media;
- with mitotane and other inhibitors of the function of the adrenal cortex – may cause an increase in the dose of the drug;
with antiemetics – increased antiemetic effect; - with isoniazid, mexiletine, praziquantel – a decrease in their plasma concentrations;
- with somatotropin (in high doses) – a decrease in the effect of the latter;
- with erythromycin – may inhibit the metabolism of certain corticosteroids;
- with fluoroquinolones – tendon damage;
- with retinoids and tetracyclines – an increase in intracranial pressure is possible;
- with cyclosporine – increases plasma concentrations of prednisolone. The same effect is possible with ritonavir. There have been cases of seizures. Since the simultaneous administration of these drugs causes a mutual slowdown in metabolism, it is likely that convulsions and other side effects associated with the use of each of these drugs, both in monotherapy and when they are used together, may occur more often. Co-administration may cause an increase in plasma concentrations of other medicinal products.
With long-term therapy, prednisolone increases the content of folic acid.
The drug reduces the effect of vitamin D on the absorption of Ca2 + in the intestinal cavity.
Precautions and Warnings of Prednisolone 30 mg/ml:
Anti-inflammatory/immunosuppressive effects and infection.
With extreme caution prescribed for immunodeficiency states (including AIDS or HIV infection).
Drug treatment, even at low doses, masks the signs and symptoms of pre-existing infections and those developed during treatment (including opportunistic infections) and makes them difficult to diagnose. Therefore, during treatment, contact with patients with a cold or other infections should be avoided.
Suppression of the inflammatory response and immune function increases susceptibility to infections and the severity of their course. When they are used, new infections may appear. The resulting opportunistic infections can be fatal. The clinical picture can often be atypical, and serious infections (such as septicemia and tuberculosis) can be masked and diagnosed already in an advanced stage.
In infectious diseases and latent forms of tuberculosis, the drug should be prescribed only in combination with antibiotics and anti-tuberculosis drugs.
Chickenpox is of particular concern as this minor illness can be fatal in immunosuppressed patients. Patients (or parents of children) who have not previously had chickenpox should be advised to avoid close personal contact with patients with chickenpox or herpes zoster, and should seek prompt medical attention if they are exposed. Varicella zoster immune globulin (VZIG) passive immunization is required for non-immune patients who are receiving systemic corticosteroids or who have used them within the previous 3 months.
Patients who during the treatment period were in contact with patients with measles or chicken pox should be given specific immunoglobulins as a prophylaxis (within 10 days after contact). Special care and urgent treatment is needed in case of confirmation of the diagnosis of chickenpox. Corticosteroids should not be discontinued and the dose may be increased.
During treatment, immunization with live vaccines should not be carried out in persons with impaired immune systems. Inactivated (killed) vaccines or toxoids can be used, although their effects may be reduced.
During long-term therapy of any intercurrent disease, trauma or surgical procedure, it is necessary to temporarily increase the dosage. If the use of corticosteroids is completed after long-term therapy, it may be necessary to temporarily re-use them. With intercurrent infections, septic conditions, it is necessary to simultaneously carry out antibiotic therapy.
Application for severe infectious diseases (not listed in the “Contraindications” section) is possible only against the background of specific antimicrobial therapy.
Chronic immunosuppression (eg, in the setting of organ transplantation) has been associated with an increased risk of malignancy.
Cancel application.
In patients receiving higher physiological doses of prednisolone (approximately 7.5 mg prednisolone or equivalent) for more than 3 weeks, prednisolone treatment should be stopped gradually. Treatment should be stopped gradually, even if it lasted less than 3 weeks, for such groups of patients:
- patients who are undergoing a second course of treatment with prednisolone;
- patients who received a second course of treatment within a year after long-term treatment (months, years);
- patients who receive more than 40 mg/day of prednisolone or equivalent;
- patients with adrenal insufficiency not caused by exogenous corticosteroids;
- patients who have taken multiple doses of corticosteroids in the evening.
After discontinuation of treatment, withdrawal syndrome, adrenal insufficiency, as well as an exacerbation of the disease, due to which prednisolone was prescribed, may occur. If after the end of treatment with prednisolone there is functional insufficiency of the adrenal glands, the use of the drug should be resumed immediately, and the dose reduction should be carried out very slowly and with caution (for example, the daily dose should be reduced by 2-3 mg within 7-10 days). After reaching a daily dose of 7.5 mg of prednisolone, dose reduction should be slower so that the hypothalamic-pituitary-adrenal system can recover.
Abrupt discontinuation of systemic corticosteroid treatment for up to 3 weeks is appropriate if relapse is not expected. Abrupt withdrawal of up to 40 mg daily prednisone or equivalent within 3 weeks is unlikely to result in clinical depression of the hypothalamic-pituitary-adrenal system in most patients.
Atrophy of the adrenal cortex develops with long-term therapy and may persist for many years after discontinuation of treatment.
Steroid-induced secondary adrenal insufficiency can be reduced to a minimum due to gradual dose reduction. This type of insufficiency may persist for several months after the end of therapy, therefore, in any stressful situation that has arisen during this period, it is necessary to restore corticosteroid therapy.
With sudden withdrawal, especially in the case of previous use of high doses, a withdrawal syndrome occurs, which is manifested by fever, loss of appetite, nausea, vomiting, diarrhea, lethargy, dizziness, generalized musculoskeletal pain, asthenia.
Violation of vision.
Visual disturbances may occur with systemic and local use of corticosteroids. If the patient has symptoms of visual impairment or other visual disturbances, the patient should be referred to an ophthalmologist to evaluate possible causes, which may include cataracts, glaucoma, or rare diseases (such as central serous chorioretinopathy (CSCR) reported after systemic and topical corticosteroids) .
Scleroderma renal crisis.
Caution is needed in the treatment of patients with systemic sclerosis due to the increased frequency of (possibly fatal) renal crisis with hypertension and decreased urinary output, as observed with a daily dose of 15 mg or more of prednisolone. Therefore, blood pressure and kidney function (s-creatinine) should be checked regularly. If a renal crisis is suspected, blood pressure should be closely monitored.
Elderly patients.
Common side effects of systemic corticosteroids may be associated with more serious consequences in the elderly, especially osteoporosis, hypertension, hypokalemia, diabetes mellitus, infection susceptibility, and thinning of the skin. Careful clinical monitoring is necessary to avoid life-threatening reactions.
Pediatric population.
Since corticosteroids cause growth retardation in children and adolescents, which may be irreversible – use only if absolutely indicated and under the most careful supervision of a physician. Treatment should be limited to the lowest dose for a short time to minimize hypothalamic-pituitary-adrenal depression and growth retardation.
Important information about excipients.
This medicinal product contains sodium and therefore should be used with caution in patients on a sodium controlled diet.
This medicinal product contains propylene glycol which may cause symptoms similar to alcohol.
Use during pregnancy or lactation.
Pregnancy.
The ability of corticosteroids to cross the placenta varies between individual drugs. However, 88% of prednisolone is inactivated when passing the trans-placental barrier of the placenta.
Administration of corticosteroids to pregnant animals may result in fetal developmental disorders, including cleft palate (cleft palate), intrauterine growth retardation, and effects on brain growth and development. There is no evidence that corticosteroids increase the incidence of congenital anomalies such as cleft palate/lip in humans. However, when used over a long period or repeatedly during pregnancy, corticosteroids may increase the risk of intrauterine growth retardation. Hypoadrenalism can theoretically occur in newborns after prenatal exposure to corticosteroids, but usually resolves spontaneously after birth and is rarely clinically important. As with all medicines, corticosteroids should only be given when the benefits to mother and child outweigh the risks. However, when corticosteroids are needed, patients with normal pregnancies can be treated as if they were not pregnant.
Patients with preeclampsia or fluid retention need close monitoring.
Breast-feeding.
If necessary, the use of the drug breast-feeding should be discontinued.
Corticosteroids are excreted in small amounts into breast milk. However, doses up to 40 mg daily of prednisone are unlikely to cause systemic effects in an infant. In infants of mothers who take higher doses, adrenal suppression is possible, but the benefits of breastfeeding may outweigh any theoretical risk. Monitoring of the infant for adrenal suppression is recommended.
Overdose:
In case of an overdose, nausea, vomiting, bradycardia, arrhythmia, increased symptoms of heart failure, cardiac arrest, hypokalemia, increased blood pressure, muscle cramps, hyperglycemia, thromboembolism, acute psychosis, dizziness, headache, development of symptoms of hypercortisolism (weight gain, development edema, arterial hypertension, glucosuria, hypokalemia). In children with an overdose, inhibition of the hypothalamic-pituitary-adrenal system, Itsenko-Cushing’s syndrome, decreased excretion of growth hormone, and increased intracranial pressure are possible.
Treatment: discontinuation of the drug, symptomatic therapy, if necessary, correction of the electrolyte balance. There is no specific antidote.
Side Effects:
The development of severe adverse reactions depends on the dose and duration of treatment. Adverse reactions usually develop with prolonged treatment with the drug, for a short period the risk of their occurrence is unlikely.
The following side effects may be associated with long-term systemic use of corticosteroids with this frequency:
Frequency not known (cannot be estimated from available data).
On the part of the organs of vision: increased intraocular pressure with possible damage to the optic nerve, glaucoma, papilloedema, edema of the optic nerve head, posterior subcapsular cataract, central serous chorioretinopathy, thinning of the cornea and sclera, trophic changes in the cornea, exacerbation of eye viral and fungal infections, exophthalmos, blurriness vision.
From the gastrointestinal tract: nausea, vomiting, flatulence, unpleasant aftertaste in the mouth, dyspepsia, increased or decreased appetite, hiccups, epigastric pain, diarrhea, erosive esophagitis, peptic ulcers with perforation and bleeding, esophageal ulcer, esophageal candidiasis, pancreatitis , perforation of the gallbladder, gastric bleeding, local ileitis and ulcerative colitis.
On the part of the liver and biliary tract: during the period of drug use, there may be an increase in ALT, AST and alkaline phosphatase, which, as a rule, is not important and is reversible after drug withdrawal.
From the side of the kidneys and urinary system: increased risk of urates, uroliths, an increase in the number of leukocytes and erythrocytes in the urine without existing kidney damage, leukocyturia, scleroderma renal crisis *.
On the part of the endocrine system: decreased glucose tolerance, “steroidal” diabetes mellitus or manifestation of latent diabetes mellitus, increased need for insulin and oral hypoglycemic drugs, hyperlipidemia, depression of the hypothalamic-pituitary-adrenal system, growth retardation in children and adolescents, delayed sexual development in children, menstrual disorders, impaired production of sex hormones (amenorrhea), postmenopausal bleeding, Itsenko-Cushing syndrome (moon face, pituitary-type obesity, hirsutism, increased blood pressure, dysmenorrhea, amenorrhea, myasthenia gravis, striae).
From the side of metabolism: negative balance of nitrogen and calcium, violation of mineral and electrolyte balance, hypokalemia, hypokalemic alkalosis, increased appetite. Side effects due to the glucocorticosteroid activity of prednisolone: fluid retention and Na + (peripheral edema), hypernatremia, hypokalemic syndrome – arrhythmia, myalgia or muscle spasm, unusual weakness and fatigue.
From the nervous system: peripheral neuropathy, paresthesia, dizziness, headache, autonomic disorders, increased intracranial pressure, which is accompanied by vomiting, cerebellar pseudotumor, convulsions.
On the part of the psyche**: irritability, delirium, disorientation, euphoria, hallucinations, depression, paranoia, nervousness, restlessness, anxiety, sleep disturbance, insomnia, suicidal tendencies, euphobia, labile mood, increased concentration, psychological dependence, mania, manic depressive psychosis, exacerbation of schizophrenia, dementia, psychosis, epileptic seizures, cognitive dysfunction (including amnesia and impaired consciousness).
From the side of the cardiovascular system: arterial hypo- or hypertension, bradycardia, arrhythmia, asystole (due to rapid administration of the drug), development or intensification of manifestations of chronic heart failure, atherosclerosis, thrombosis, vasculitis, peripheral edema, ECG changes characteristic of hypokalemia . In patients with acute and subacute myocardial infarction – the spread of necrosis, slowing down the formation of scar tissue, which can lead to rupture of the heart muscle.
On the part of the blood and lymphatic system: an increase in the total number of leukocytes with a decrease in the number of eosinophils, monocytes and lymphocytes. The mass of lymphoid tissue decreases. Blood clotting may increase, hypercoagulability, which leads to thrombosis, thromboembolism.
From the immune system: hypersensitivity reactions, which include rash, skin itching, urticaria, flushing, angioedema, anaphylactic shock.
On the part of the skin and subcutaneous tissue: slowing down the regeneration process, petechiae, bruises, hematomas, ecchymosis, striae, thinning of the skin, hyper- or hypopigmentation, acne, a tendency to develop pyoderma, skin atrophy, telangiectasia, hyperhidrosis, acne, hirsutism, purpura, post-steroid panniculitis, which is characterized by the appearance of erythematosis, hot subcutaneous thickening for 2 weeks after discontinuation of the drug, Kaposi’s sarcoma.
From the musculoskeletal system and connective tissue: growth retardation and ossification processes in children (premature closure of epiphyseal growth zones), proximal myopathy, osteoporosis, myalgia, tendon and muscle rupture, muscle weakness, increase or decrease in muscle mass (atrophy), steroid myopathy, fractures of the spine and long bones, aseptic osteonecrosis, pathological bone fractures.
Infections and invasions: increased susceptibility and severity of infections with masking of clinical symptoms and signs of bacterial, viral, fungal infections, opportunistic infections, relapse of tuberculosis.
General disorders: malaise, persistent hiccups when using the drug in high doses, adrenal insufficiency, which leads to arterial hypotension, hypoglycemia and death in stressful situations such as surgery, trauma or infection, if the dose of prednisolone is not increased.
Reactions at the injection site: pain, burning, pigmentation changes (depigmentation, leukoderma), skin atrophy, sterile abscesses, rarely lipoatrophy.
* Scleroderma renal crisis.
Among various subpopulations, scleroderma renal crisis occurs. The greatest risk was observed in patients with diffuse systemic sclerosis. The lowest risk was registered in patients with limited systemic sclerosis (2%) and juvenile systemic sclerosis (1%).
**Reactions are common and can occur in both adults and children. In adults, the frequency of severe reactions is estimated at 5-6%. Psychological effects have been reported on withdrawal of corticosteroids; frequency is unknown.
Withdrawal symptoms.
With a sharp withdrawal of the drug, a withdrawal syndrome is possible, the severity of symptoms depends on the degree of atrophy of the adrenal glands, headache, nausea, vomiting, abdominal pain, dizziness, anorexia, weakness, mood changes, lethargy, fever, myalgia, arthralgia, rhinitis, eczema, conjunctivitis, painful itching of the skin, weight loss. In more severe cases – severe mental disorders and hypotension, increased intracranial pressure, edema of the optic disc caused by cerebral edema, steroid pseudorheumatism in patients with rheumatism, deaths.
In some cases, withdrawal symptoms may have or resemble a clinical relapse of the disease for which the patient is being treated.
Pediatric population.
Increased intracranial pressure with swelling of the optic nerve head in children (pseudotumor of the brain) – usually occurs after discontinuation of treatment. Growth retardation in children and adolescents.
Storage:
Store in the original packaging, in the refrigerator (at a temperature of 2 °C to 8 °C). Keep out of the reach of children.
Shelf life:
2 year.




Reviews
There are no reviews yet.