Description
Buy Ovitrelle 250 mg/0.5 ml prefilled syringe
Description of Ovitrelle 250 mg/0.5 ml prefilled syringe:
It is used in a complex of assisted reproductive technologies (including for in vitro fertilization) to induce the final maturation of follicles and luteinization after stimulation with gonadotropins.
Ingredients:
Active Ingredient:
Pharmacodynamic Properties of Ovitrelle 250 mg/0.5 ml prefilled syringe:
Ovitrelle is a medicinal product of human hCG produced by recombinant DNA technology. HCG alfa shares an amino acid sequence with human chorionic gonadotropin (hCG) isolated from urine. On cells of the ovarian folder (and granulosa), human chorionic gonadotropin binds to transmembrane LH/CG receptors, which also bind luteinizing hormone (LH).
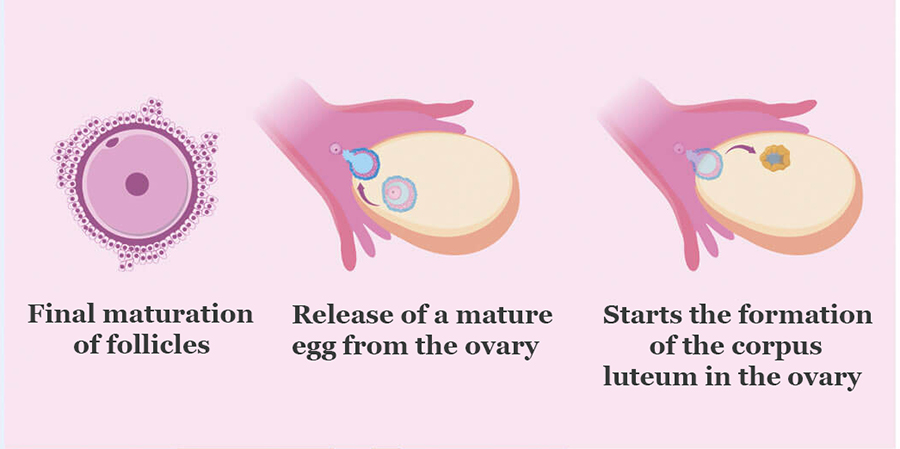
The main pharmacodynamic action of the drug in women is the renewal of oocyte meiosis, rupture of the follicle (ovulation), the formation of the corpus luteum and the production of progesterone and estradiol by the corpus luteum. In women, human chorionic gonadotropin acts like an LH surge that triggers ovulation.
Ovitrelle is used to initiate the final maturation of follicles and early luteinization after the use of drugs that stimulate follicular growth. In comparative clinical studies, administration of Ovitrelle 250 mcg was as effective as administration of 5,000 IU or 10,000 IU of urinary hCG in inducing final follicular maturation and early luteinization when assisted reproductive technology (GRT 5) was used to induce ovulation
So far, no signs of developing antibodies to Ovitrelle® have been found in humans. Repeated use of Ovitrelle has only been studied in men. A clinical study of the use of the drug in women during ART and anovulation was limited to one treatment cycle.
Pharmacokinetics Properties of Ovitrelle 250 mg/0.5 ml prefilled syringe:
After s / c administration, absolute bioavailability is about 40%, T1 / 2 is about 30 hours.
Indications for use of Ovitrelle 250 mg/0.5 ml prefilled syringe:
It is used in a complex of assisted reproductive technologies (including for in vitro fertilization) to induce the final maturation of follicles and luteinization after stimulation with gonadotropins.
With anovulatory or oligoovulatory infertility for the induction of ovulation and luteinization at the end of follicular growth stimulation.
Contraindications of Ovitrelle 250 mg/0.5 ml prefilled syringe:
Due to the risk of side effects, Ovitrelle is contraindicated in some patients.
The main contraindications to the use of the drug include:
- allergy to the main or auxiliary substance;
- benign and malignant tumors of the ovaries, uterus, mammary glands, hypothalamus and pituitary gland;
- vaginal bleeding of unknown origin;
- the presence of cysts in the ovaries, except for those caused by Stein-Leventhal syndrome;
- ectopic pregnancy diagnosed in the last three months;
- disruption of the blood coagulation system.
In addition, it is undesirable to use the drug in cases where it is known to be of low effectiveness, or if a woman has contraindications for carrying a pregnancy (primary ovarian failure, anomalies in the development of the organs of the reproductive system, uterine tumors that are incompatible with pregnancy).
Side effects of Ovitrelle 250 mg/0.5 ml prefilled syringe:
Reception of Ovitrella may be accompanied by undesirable effects.
The most common of them:
- headache;
- fatigue;
- general weakness;
- redness and soreness at the injection site;
- puffiness;
- stomach ache;
- nausea;
- diarrhea;
- pain in the mammary gland;
- ovarian hyperstimulation syndrome.
Interaction with other medicinal products:
No specific drug interaction studies have been conducted with Ovitrelle, but no clinically significant drug interactions have been observed during therapy with hCG.
Overdose:
The effects of an overdose of Ovitrelle are unknown. However, due to overdose, there is a possibility of developing OHSS.
Storage:
Store at 2-8°C (refrigerated). Store in original packaging to protect from light. Keep out of the reach of children.
During the expiration date, the drug can be stored at a temperature not exceeding 25 C for up to 30 days without re-refrigeration. If the solution has not been used within 30 days, it should be discarded.
Shelf life:
2 years.
Package:
0.5 ml solution for injection in a pre-filled syringe (type I glass) with a piston stopper (halobutyl rubber), a plastic piston rod and a fixed stainless steel needle closed with a combination cap (rubber/polypropylene). One pre-filled syringe in a blister pack is placed in a cardboard box.







Reviews
There are no reviews yet.