Description
Buy Nimesil granules for oral suspension 100 mg 2 g #30
Description of Nimesil granules for oral suspension:
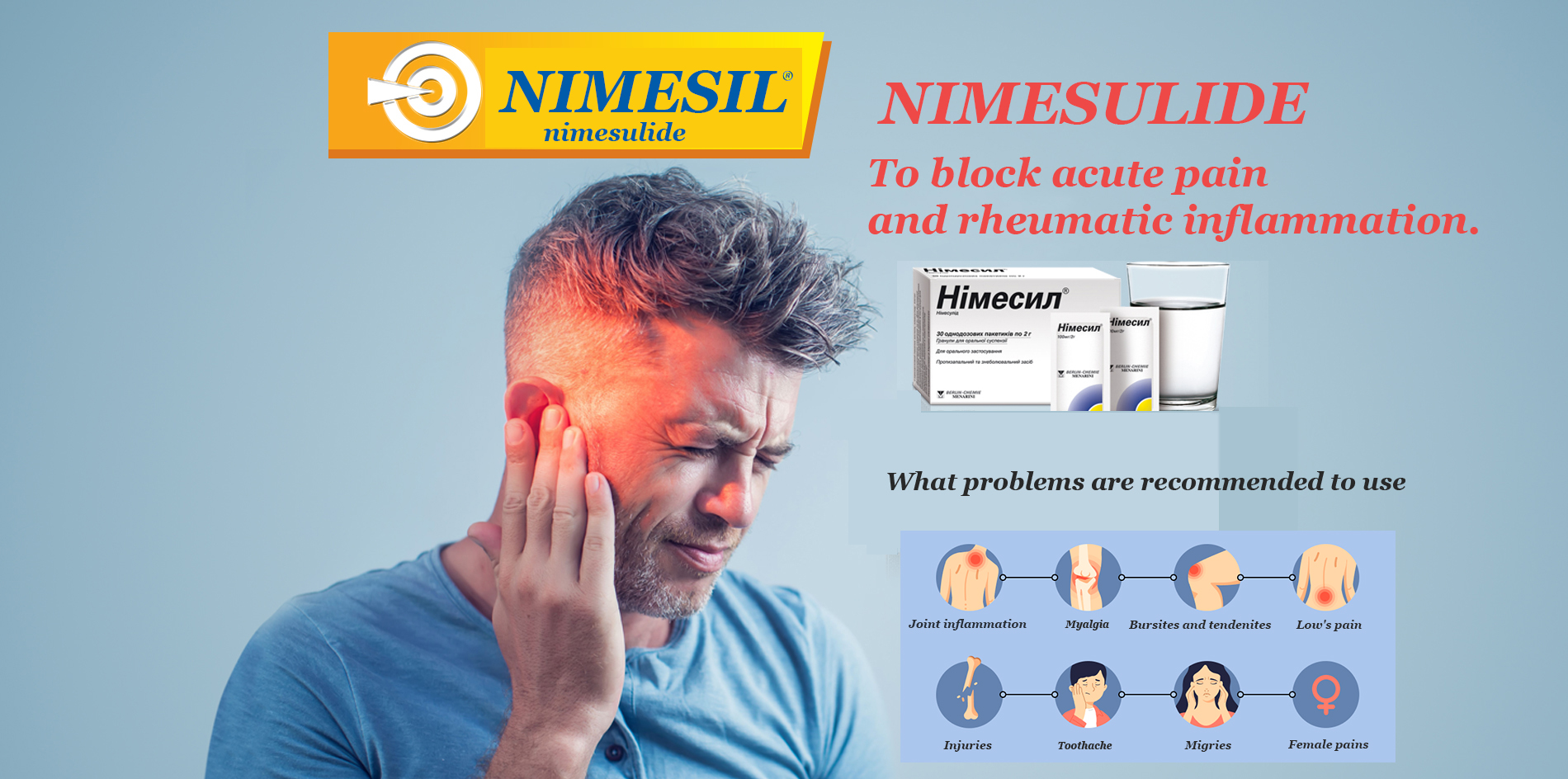
Nimesil contains nimesulide as an active ingredient – NSAIDs with predominant inhibitory activity against the COX-2 enzyme. Nimesulide has a complex mechanism of action, including the reduction of free radical formation and other components of neutrophil activation, which further explains its anti-inflammatory activity.
Nimesulide has powerful analgesic, anti-inflammatory and antipyretic properties with a relatively low risk of side effects from the gastrointestinal tract, as evidenced by the results of numerous clinical studies. In addition, when administered orally, it is rapidly absorbed, which makes it possible to effectively stop acute pain.
Nimesulide exhibits therapeutic efficacy in a diverse range of diseases that have an inflammatory component in the pathogenesis and are manifested by pain syndrome. So, it significantly alleviates the severity of the symptoms of rheumatoid arthritis and osteoarthritis, reducing the intensity of pain both at rest and during movement, increasing the range of motion and reducing the severity and duration of morning stiffness, as well as pain on palpation of the affected joints.
Ingredients:
Active substance: nimesulide;
1 single package of 2 g of granules contains nimesulide 100 mg;
Excipients: macrogol cetostearyl ether, sucrose, maltodextrin, anhydrous citric acid, orange flavor.
Pharmacodynamics of Nimesil granules for oral suspension:
Nimesulide is an NSAID with analgesic and antipyretic properties that acts as an inhibitor of the COX enzyme responsible for the synthesis of prostaglandins.
Pharmacokinetics of Nimesil granules for oral suspension:
Suction. Nimesulide is well absorbed when taken orally. After taking a single dose of 100 mg of nimesulide, in adults, Cmax in blood plasma is reached after 2-3 hours and is 3-4 mg / l. AUC – 20-35 mg h / l. There was not a single statistically significant difference between these indicators and the same indicators after taking 100 mg 2p / day for 7 days. Up to 97.5% of nimesulide binds to plasma proteins.
Metabolism and elimination. Nimesulide is actively metabolized in the liver in various ways, including with the participation of the cytochrome P450 CYP2C9 enzyme. Therefore, there is a risk of drug interactions when used simultaneously with drugs that are metabolized with the participation of CYP 2C9 (see INTERACTIONS). The main metabolite is a parahydro derivative, which is also pharmacologically active. The period before the detection of this metabolite in the circulating blood is short (≈0.8 h), but the reaction constant of its formation is low and significantly less than the absorption coefficient of nimesulide. Hydroxynimesulide is the only metabolite detected in plasma and is almost entirely in the bound form. T½ – 3.2-6x. Nimesulide is excreted mainly in the urine (≈50% of the dose taken). Only 1-3% is excreted unchanged. Hydroxynimesulide is the main metabolite, which is determined exclusively as glucuronate. About 29% of the dose taken is excreted in the feces as metabolites. The pharmacokinetic profile of nimesulide in elderly patients does not change with single and repeated administration.
Warnings and Safety for use of Nimesil granules for oral suspension:
Preclinical data obtained in standard studies of pharmacological safety, repeated dose toxicity, genotoxicity and carcinogenicity did not reveal any particular danger to humans. In repeat dose toxicity studies, nimesulide has shown gastrointestinal, renal, and hepatic toxicity. In studies of reproductive toxicity when the drug was administered to females at doses that did not have a toxic effect, embryotoxic and teratogenic effects (malformations of the skeleton, dilatation of the ventricles of the brain) were observed in rabbits, but not in rats. In rats, increased mortality of offspring in the early postnatal period and adverse reactions regarding fertility were observed.
Indications for use of Nimesil granules for oral suspension:
Treatment of acute pain, primary dysmenorrhea.
Nimesulide should only be used as a second line drug.
The decision to prescribe nimesulide should be made on the basis of an assessment of all risks for a particular patient.
Contraindications of Nimesil granules for oral suspension:
- Hypersensitivity to nimesulide, to any other NSAID or to any of the excipients of the drug;
- Hyperergic reactions in history (for example, bronchospasm, rhinitis, urticaria) in connection with the use of acetylsalicylic acid or other non-steroidal anti-inflammatory drugs;
- History of hepatotoxic reactions to nimesulide;
- Concomitant use of other substances with potential hepatotoxicity;
- Alcoholism and drug addiction;
- Gastrointestinal bleeding or perforation in history associated with previous use of NSAIDs;
- Peptic ulcer in the acute phase or a history of bleeding in the digestive tract, ulcers or perforations;
- Cerebrovascular bleeding or other hemorrhages, as well as diseases accompanied by bleeding;
- Severe bleeding disorders;
- Severe heart failure;
- Severe impairment of kidney function;
- Impaired liver function;
- Fever and/or flu-like symptoms;
- Age up to 12 years;
- Third trimester of pregnancy and lactation.
Interactions:
Corticosteroids. Corticosteroids may increase the risk of gastrointestinal ulcers or bleeding.
Antiplatelet agents and selective serotonin reuptake inhibitors (SSRIs). Antiplatelet agents and selective serotonin reuptake inhibitors (SSRIs) increase the risk of ulcers or bleeding in the digestive tract.
Anticoagulants. NSAIDs can enhance the effects of anticoagulants such as warfarin. When treating patients with nimesulide taking warfarin or similar anticoagulants or acetylsalicylic acid, there is an increased risk of complications in the form of bleeding, so this combination is not recommended and is contraindicated in patients with severe coagulation disorders. If combination therapy cannot be avoided, careful monitoring of blood coagulation parameters should be carried out.
Diuretics, angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II antagonists (AA II). NSAIDs can weaken the effect of diuretics and other antihypertensive drugs. In some patients with reduced renal function (in dehydrated patients or elderly patients with impaired renal function), the combined use of ACE inhibitors and cyclooxygenase inhibitors may cause further deterioration of renal function, including the occurrence of acute renal failure, which is usually reversible. The occurrence of such interactions should be considered in patients who have to use drugs containing nimesulide, together with ACE inhibitors or AA II. Therefore, this combination of drugs should be used with caution, especially in elderly patients. Patients should receive sufficient fluids. The need to monitor renal function after the start of concomitant treatment and periodic monitoring after its termination should be analyzed.
Other non-steroidal anti-inflammatory drugs (NSAIDs). The combined use of drugs containing nimesulide, with other NSAIDs, including acetylsalicylic acid in anti-inflammatory doses (≥ 1 g as a single dose or ≥ 3 g per day), is not recommended.
Furosemide. In healthy volunteers, nimesulide temporarily weakens the effect of furosemide in sodium excretion and, to a lesser extent, in potassium excretion, and reduces the diuretic effect. The simultaneous use of nimesulide and furosemide leads to a decrease (by approximately 20%) in the area under the concentration-time curve (AUC) and the cumulative excretion of furosemide without changes in renal clearance. The combined use of furosemide and drugs containing nimesulide in patients with impaired renal or cardiac function requires caution.
Lithium. There have been reports that NSAIDs reduce lithium clearance, leading to increased plasma levels and lithium toxicity. When prescribing nimesulide to a patient who is receiving lithium therapy, plasma lithium levels should be carefully monitored.
Pharmacokinetic interactions: the effect of other drugs on the pharmacokinetics of nimesulide.
In vitro studies have shown that nimesulide is displaced from the binding sites by tolbutamide, salicylic acid and valproic acid. However, despite the possible effect on its concentration in blood plasma, such an interaction has no clinical significance.
Other interactions.
Possible pharmacokinetic interactions with glibenclamide, theophylline, warfarin, digoxin, cimetidine and antacids (namely a combination of aluminum and magnesium hydroxide) have also been investigated in vivo. No clinically significant interactions were observed.
Nimesulide inhibits the activity of the CYP2C9 enzyme. Plasma concentrations of drugs that are substrates of this enzyme may increase with the simultaneous use of the drug Nimesil®.
Care must be taken when nimesulide is used less than 24 hours before or less than 24 hours after taking methotrexate, since it is possible to increase the level of the latter in the blood serum and increase its toxicity.
In connection with the effect on renal prostaglandins, prostaglandin synthetase inhibitors such as nimesulide may increase the nephrotoxicity of cyclosporine.
Application Features
Adverse reactions can be minimized by using the lowest effective dose for the shortest period of time necessary to control symptoms.
If treatment is not effective, therapy should be discontinued.
The combined use of nimesulide with NSAIDs, including selective cyclooxygenase-2 inhibitors, should be avoided. During therapy with Nimesil®, patients should be advised to refrain from using other analgesics.
Nimesil® contains sucrose. Patients with rare hereditary fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase deficiency should not take this drug.
During treatment with nimesulide, it is recommended to avoid the simultaneous use of hepatotoxic drugs and to refrain from drinking alcohol. The use of NSAIDs may mask fever associated with an underlying bacterial infection.
Effect on the liver.
Serious liver reactions associated with the use of nimesulide have been rarely reported, including very rarely fatal cases. Patients who, during treatment with nimesulide, experience symptoms similar to those of liver damage, for example, anorexia, nausea, vomiting, abdominal pain, fatigue, dark urine, or patients in whom laboratory tests of liver function deviate from normal values, should stop therapy. Such patients should not be re-assigned nimesulide. Liver damage, in most cases reversible, has been reported after short-term exposure to the drug.
Patients taking nimesulide and who develop fever and/or flu-like symptoms should stop treatment.
Influence on the gastrointestinal tract.
Bleeding or ulcers/perforations in the gastrointestinal tract (with or without warning symptoms or a history of serious gastrointestinal events) have been reported, which could be fatal and occur at any time during treatment with all NSAIDs. The risk of gastrointestinal bleeding, ulceration or perforation increases with increasing doses of NSAIDs in patients with a history of ulcer, especially when it is complicated by bleeding or perforation, and in elderly patients. In such patients, treatment should be initiated at the lowest possible dose. For these patients, and for those requiring concomitant use of low doses of acetylsalicylic acid or other drugs that increase the risk of gastrointestinal complications, combination therapy with protective agents such as misoprostol or proton pump inhibitors should be considered.
Patients with a history of gastrointestinal toxicity, especially elderly patients, should report any unusual abdominal symptoms (especially gastrointestinal bleeding), especially in the initial stages of treatment.
The appearance of bleeding or ulcers / perforations in the digestive tract is possible at any time during treatment with or without warning symptoms or a history of gastrointestinal events. If bleeding or ulceration occurs in the gastrointestinal tract, the use of nimesulide should be discontinued. Nimesulide should be used with caution in patients with gastrointestinal disorders, including peptic ulcer, gastrointestinal bleeding, ulcerative colitis, or a history of Crohn’s disease.
Patients taking concomitant medications that may increase the risk of ulcers or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors, or antiplatelet agents such as acetylsalicylic acid, should be advised to exercise caution.
In the event of bleeding or ulceration of the digestive tract in patients receiving nimesulide, treatment should be discontinued.
NSAIDs should be used with caution in patients with a history of gastrointestinal disease (ulcerative colitis, Crohn’s disease), as it may worsen.
Simultaneous use of nimesulide with other drugs, such as oral contraceptives, anticoagulants, antiplatelet agents, may exacerbate Crohn’s disease and other diseases of the digestive tract.
Influence on the cardiovascular and cerebrovascular systems.
Patients with arterial hypertension and / or congestive heart failure of mild or moderate severity in history require appropriate monitoring and consultation with a physician, since fluid retention and edema have been reported as a result of NSAID therapy.
Clinical studies and epidemiological data suggest that the use of some NSAIDs, especially at high doses and in long-term treatment, may be associated with a modest increase in the risk of arterial thrombotic events such as myocardial infarction or stroke. There are not enough data to exclude such a risk when using nimesulide.
Patients with uncontrolled arterial hypertension, congestive heart failure, established coronary heart disease, peripheral arterial disease and / or cerebrovascular disease should be treated with nimesulide only after a thorough assessment of the condition. Such an assessment should be carried out before starting long-term treatment in patients with risk factors for the development of cardiovascular diseases, for example, with arterial hypertension, hyperlipidemia, diabetes mellitus, and smoking.
Since nimesulide can affect platelet function, it should be used with caution in patients with hemorrhagic diathesis. However, the drug Nimesil® cannot replace acetylsalicylic acid in the prevention of cardiovascular diseases.
Effect on the kidneys.
Patients with impaired renal function or heart failure should be careful, since the use of nimesulide may lead to deterioration of renal function. In case of deterioration, treatment should be discontinued.
Elderly patients.
In elderly patients, the incidence of adverse reactions to NSAIDs, especially bleeding and perforation in the digestive tract, in some cases even fatal, as well as impaired renal, cardiac and liver function, may be increased, therefore appropriate clinical monitoring is recommended.
Skin reactions.
Very rarely, serious skin reactions, some of which are fatal, have been reported, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis associated with NSAIDs. Obviously, patients are most at risk of such reactions at the beginning of the course of treatment, in most cases reactions appear during the first month of therapy. Nimesulide should be discontinued at the first appearance of signs of skin rash, mucosal lesions or any other manifestations of hypersensitivity.
When using nimesulide, cases of fixed drug rash (FMR) have been reported. Nimesulide should not be re-administered to patients with a history of nimesulide-related cases of PMF.
Impact on fertility.
The use of the drug Nimesil® may interfere with female fertility and is not recommended for women planning pregnancy. Women who find it difficult to get pregnant or who are being examined for infertility should consider the difference between Nimesil®.
Overdose of Nimesil granules for oral suspension:
Symptoms of acute NSAID overdose are usually limited to the following: apathy, drowsiness, nausea, vomiting and epigastric pain, which are usually reversible with maintenance therapy. Gastrointestinal bleeding may occur. Rarely, hypertension, acute renal failure, respiratory depression and coma are possible. There have been reports of anaphylactoid reactions with therapeutic doses of NSAIDs, which may occur in overdose. In case of NSAID overdose, patients should receive symptomatic and supportive treatment. There are no specific antidotes. There is no information on the elimination of nimesulide by hemodialysis, but given its high degree of binding to plasma proteins (up to 97.5%), it is unlikely that dialysis will be effective in case of overdose. If symptoms are present or after a severe overdose, patients may be given vomiting and/or activated charcoal (60–100 g for adults) and/or an osmotic laxative within 4 hours of taking the drug. Forced diuresis, urine retention, hemodialysis, or hemoperfusion may not be effective due to high protein binding. Kidney and liver functions should be monitored.
Side effects:
The most common adverse reactions are from the digestive tract. Perhaps the occurrence of peptic ulcers, perforation or bleeding in the digestive tract, sometimes life-threatening, especially in elderly patients. Nausea, vomiting, diarrhea, bloating, constipation, dyspepsia, abdominal pain, melena, hematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn’s disease have been reported after the use of drugs containing nimesulide. Rarely observed gastritis. There have been reports of edema, hypertension and heart failure in association with NSAID treatment. Very rarely, bullous reactions have been reported, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Clinical and epidemiological studies indicate that the use of some NSAIDs, especially at high doses and during long-term treatment, may cause a slight increase in the risk of arterial thrombotic complications, such as myocardial infarction or stroke.
Storage:
Store below 25°C in original packaging. Keep out of the reach of children.
Shelf life:
3 years.




Reviews
There are no reviews yet.