Description
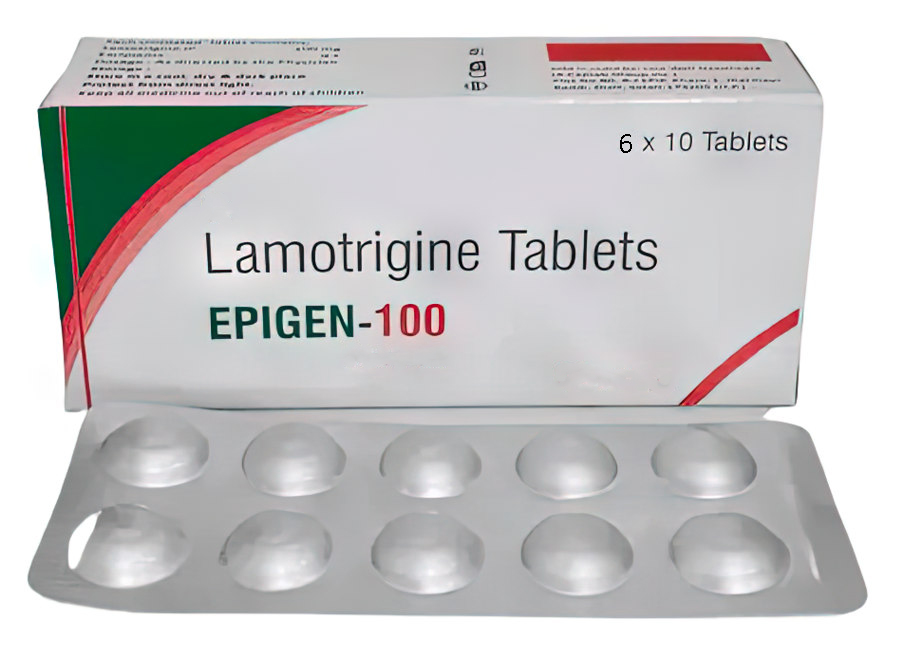
Buy Lamotrin Lamotrigine tablets 100 mg # 60
Description of Lamotrin Lamotrigine 100 mg # 60:
Lamotrigine, a phenyltriazine derivative, is an antiepileptic drug whose mechanism of action is associated with the blockade of voltage-gated sodium channels of the presynaptic membranes of neurons in the delayed inactivation phase and inhibition of the excessive release of excitatory neurotransmitters, primarily glutamate (an amino acid that plays a significant role in the development of an epileptic attack).
Active substance: 1 tablet contains lamotrigine 100 mg;
Excipients: microcrystalline cellulose, colloidal anhydrous silica, povidone, sodium starch glycolate (type A), lactose monohydrate, magnesium stearate.
Pharmacodynamics of Lamotrin Lamotrigine 100 mg:
Lamotrigine is an anticonvulsant drug, the mechanism of action of which is associated with the blockade of voltage-gated sodium channels of the presynaptic membranes of neurons in the slow inactivation phase and inhibition of excessive release of glutamate (an amino acid that plays a significant role in the development of an epileptic attack).
Pharmacokinetics of Lamotrin Lamotrigine 100 mg:
After oral administration, the drug is quickly and completely absorbed from the gastrointestinal tract. The maximum concentration in blood plasma is reached after 2.5 hours.
Lamotrigine is extensively metabolized, the main metabolite being N-glucuronide. In adults, the half-life averages 29 hours. Lamotrigine has a linear pharmacological profile. It is excreted mainly in the form of metabolites and partly unchanged, mainly in the urine. In children, the half-life is shorter than in adults.
Special groups of patients.
Children.
Clearance depending on body weight in children is higher than in adults, the highest rates are in children under 5 years of age. The half-life of lamotrigine in children is generally shorter than in adults, with a mean of approximately 7 hours when coadministered with enzyme inducers such as carbamazepine and phenytoin, and an increase in the mean to 45-50 hours when coadministered exclusively with valproate. .
Elderly patients.
It was reported that the results of a pharmacokinetic analysis in patient groups that included both elderly and young patients with epilepsy participating in one study found that the clearance of lamotrigine was not changed by a clinically significant measure. After single doses, apparent clearance decreased by 12% from 35 ml/min/kg in patients aged 20 years to 31 ml/min/kg in patients aged 70 years. The reduction after 48 weeks of treatment was 10%, from 41 ml/min in younger patients to 37 ml/min in older patients. It was reported that the pharmacokinetics of lamotrigine were studied in 12 healthy elderly patients who were prescribed a single dose of 150 mg. The mean clearance in elderly patients (0.39 ml/min/kg) is between the mean clearance (0.31 to 0.65 ml/min/kg) obtained in 9 studies conducted in non-elderly adults after receiving a single dose of 30 to 450 mg.
Patients with impaired renal function.
A single dose of 100 mg lamotrigine was administered to 12 volunteers with chronic renal impairment and 6 hemodialysis patients. The mean CL/F values were 0.42 ml/min/kg (chronic renal impairment), 0.33 ml/min/kg (inter-hemodialysis period) and 1.57 ml/min/kg (during hemodialysis) compared with 0.58 ml/min/kg in healthy patients. The mean plasma half-life was 42.9 hours (chronic renal impairment), 57.4 hours (inter-dialysis period) and 13.0 hours (during hemodialysis), compared with 26.2 hours in healthy patients. Over the course of a four-hour hemodialysis session, the amount of lamotrigine was reduced by approximately 20% (range 5.6 to 35.1). For this group of patients, the initial dose of lamotrigine should be based on the patient’s antiepileptic drug regimen; A reduction in maintenance dose may be effective in patients with significant functional renal impairment.
Patients with impaired liver function.
A single dose pharmacokinetic study was conducted in patients with varying degrees of liver dysfunction and healthy volunteers. The mean apparent clearance of lamotrigine was 0.31 mL/min/kg, 0.24 mL/min/kg, and 0.10 mL/min/kg in patients with Child–Pugh grade A, B, and C impairment. liver, respectively, compared with 0.34 ml/min/kg in healthy patients. Typically, initial, escalation, and maintenance doses should be reduced by approximately 50% in patients with moderate hepatic impairment (Child-Pugh grade B) and by 75% in patients with severe liver dysfunction (Child-Pugh grade C). liver.Elevated and maintenance doses should be adjusted depending on the response to treatment.
Indications for use of Lamotrin Lamotrigine 100 mg:
Epilepsy.
Adults and children over 13 years of age: monotherapy and adjunctive therapy for partial and generalized seizures of epilepsy, including tonic-clonic seizures, as well as seizures associated with Lennox-Gastaut syndrome. The drug Lamotrin is prescribed as additional therapy, but for Lennox-Gastaut syndrome it can be prescribed as an initial antiepileptic drug (AED).
Children 2 to 12 years: adjunctive therapy for epilepsy, particularly partial and generalized seizures, including tonic-clonic seizures, and seizures associated with Lennox–Gastaut syndrome.
Monotherapy for typical absence seizures.
Bipolar disorders in adults.
Adults (age 18+).
To prevent depressive conditions in patients with bipolar I disorder, who predominantly suffer from depressive conditions.
Lamotrigine is not indicated for the acute treatment of manic or depressive episodes.
Contraindications of Lamotrin Lamotrigine 100 mg:
Hypersensitivity to lamotrigine or other components of the drug.
Interactions:
Uridine 5′-diphospho (UDP)-glucuronyl transferase (UGT) has been identified as the enzyme responsible for the metabolism of lamotrigine. Therefore, drugs that induce or inhibit glucuronidation may affect the clearance of lamotrigine. Strong to moderate inducers of the cytochrome P450 3A4 (CYP3A4) enzyme, which are known to induce UGT, may also enhance the metabolism of lamotrigine. There is no evidence that lamotrigine may cause clinically significant stimulation or inhibition of cytochrome P450 oxidative enzymes. Lamotrigine may induce its metabolism, but this effect is moderate and does not have significant clinical consequences.
Several drugs have been shown to have relevant clinical effects on lamotrigine concentrations. In general, concomitant use of such drugs is not expected to result in any clinical effects. However, caution should be exercised in patients with epilepsy, whose disease state is particularly sensitive to fluctuations in lamotrigine concentrations.
Valproate – increases the concentration of lamotrigine.
Atazanovir/ritonavir, Carbamazepine, ethinylestradiol/levonorgestrel combination, Lopinavir/ritonavir, Phenobarbital, Phenytoin, Primidone, Rifampicin – reduce the concentration of lamotrigine.
Aripiprazole, Bupropion, Felbomate, Gabapentin, Lacosamide, Levetiracetam, Lithium, Olanzapine, Oxcarbazepine, Paracetamol, Perempanel, Pregabalin, Topiramate, Zonisamide – have little or no effect on the concentration of lamotrigine.
Effect of hormonal contraceptives on the pharmacokinetics of lamotrigine.
There is evidence that the combination of ethinyl estradiol 30 mcg/levonorgestrel 150 mcg increases the elimination of lamotrigine by approximately 2 times, which in turn leads to a decrease in the area under the curve AUC and Cmax of lamotrigine by an average of 52% and 39%, respectively. During a one-week break in the use of a contraceptive (the so-called contraceptive-free week), the concentration of lamotrigine in the blood serum gradually increased, reaching a concentration that was approximately 2 times higher than when the drugs were used together.
Effect of lamotrigine on the pharmacokinetics of hormonal contraceptives.
It is known that, according to studies in women, a constant dose of lamotrigine 300 mg did not affect the pharmacokinetics of ethinyl estradiol, which is part of the combination oral contraceptive tablet.
Application Features
Skin rashes
In the first 8 weeks of starting treatment with lamotrigine, an adverse skin reaction in the form of a rash may occur. In most cases, the rash is mild and resolves without treatment, but severe skin reactions requiring hospitalization and discontinuation of the drug have been reported. These include cases of potentially life-threatening rashes, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS); also known as hypersensitivity syndrome (HSS).
Patients who experience Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic manifestations (DRESS) after receiving lamotrigine should not be re-treated with lamotrigine. It is known that in adults who participated in studies following current lamotrigine dosing recommendations, the incidence of severe skin rash was approximately 1 in 500 patients with epilepsy. Approximately half of these cases were diagnosed with Stevens-Johnson syndrome (1 in 1000).
The incidence of severe skin rashes in patients with bipolar disorder is 1 in 1000.
Children are at higher risk of developing serious skin rashes than adults. It is known that, according to studies of the use of lamotrigine, the incidence of rashes leading to hospitalization in children varies from 1 in 300 to 1 in 100 patients.
In children, the first signs of a skin rash may be mistaken for an infection, so clinicians should be aware of the possibility of an adverse reaction to the drug in children who develop a rash and fever during the first 8 weeks of therapy.
The overall risk of skin rash appears to be closely related to high initial doses of lamotrigine and exceeding the recommended dose escalation schedule for lamotrigine therapy.
Caution should be exercised when prescribing lamotrigine to patients who have a history of allergies or rash while using other antiepileptic drugs, since the incidence of moderate rash after treatment with lamotrigine in this group of patients was 3 times higher than in the group without such a history.
All patients (adults and children) who develop a rash should be examined immediately by a physician and treatment with lamotrigine should be discontinued immediately, unless the development of the rash is not related to lamotrigine. It is not recommended to reinstate lamotrigine in cases where its previous prescription was canceled due to the development of skin reactions. In this case, when deciding on re-prescribing the drug, it is necessary to take into account the expected benefits of treatment and the possible risks.
The skin rash has been reported to be a manifestation of DRESS syndrome, also known as hypersensitivity syndrome. This condition is accompanied by various systemic symptoms, including fever, lymphadenopathy, facial edema, abnormal blood counts, liver and kidney dysfunction, and aseptic meningitis. The syndrome can vary in severity and can rarely lead to disseminated intravascular coagulation and multiple organ failure. Early signs of hypersensitivity (eg, fever and lymphadenopathy) may occur even in the absence of skin rash. If these symptoms occur, the patient should be assessed immediately and, unless there is other reason, discontinue lamotrigine.
In most cases, aseptic meningitis reverses after discontinuation, but in some cases it may return when lamotrigine is re-administered. Repeated administration of lamotrigine results in a rapid return of symptoms, often of a more severe nature. Patients in whom lamotrigine was discontinued due to the occurrence of aseptic meningitis when previously prescribed it should not be re-prescribed lamotrigine.
There have also been reports of photosensitivity reactions associated with the use of lamotrigine (see Adverse Reactions section). In a few cases, a reaction occurred with a high dose (400 mg or more), after a dose increase or rapid titration. If lamotrigine-related photosensitivity is suspected in a patient with signs of photosensitivity (eg, severe sunburn), discontinuation of treatment should be considered. If continued treatment with lamotrigine is considered clinically warranted, the patient should be advised to avoid exposure to sunlight and artificial ultraviolet light and to take protective measures (eg, use of protective clothing and sunscreen).
Hemophagocytic lymphohistiocytosis (HLH)
HLH has been reported in patients taking lamotrigine (see Adverse Reactions section). HLH is characterized by signs and symptoms such as fever, rash, neurological symptoms, hepatosplenomegaly, lymphadenopathy, cytopenia, increased serum ferritin concentrations, hypertriglyceridemia, and abnormal liver function and coagulation. Symptoms usually occur within 4 weeks of starting treatment.
Patients should be informed of the symptoms associated with HLH and should be advised to seek immediate medical attention if these symptoms occur during treatment with lamotrigine.
Patients who develop these signs and symptoms should be evaluated and a diagnosis of HLH considered. Lamotrigine therapy should be discontinued immediately if another cause for symptoms cannot be determined.
Suicidal risk
People with epilepsy may experience symptoms of depression and/or bipolar disorder, and there is evidence that people with epilepsy and bipolar disorder have an increased risk of suicide.
Between 25 and 50% of patients with bipolar disorder have at least one suicide attempt. They may experience worsening depressive symptoms and/or suicidal thoughts and behavior (suicidality) whether or not they have used medications to treat bipolar disorder, particularly lamotrigine.
Suicidal ideation and behavior have been reported when patients with various indications, including epilepsy, are treated with antiepileptic drugs. A meta-analysis using antiepileptic drugs, including lamotrigine, showed a nonsignificant increase in the risk of suicidal ideation and behavior. The mechanism of this risk is unknown, but available data do not exclude the possibility of an increased risk due to the use of lamotrigine. Therefore, patients should be carefully monitored for suicidal intentions and behavior. If these signs occur, patients and their caregivers should seek medical attention.
Clinical worsening in bipolar disorder
Patients taking lamotrigine for bipolar disorder should be closely monitored for clinical worsening (including the onset of new symptoms) or suicidality, especially at the start of treatment or during dose changes.
Patients who have a history of suicidal behavior or ideation, or who have demonstrated significant suicidal intent prior to treatment, are at greater risk for suicidal ideation or suicide attempts and require close monitoring during treatment.
Patients and caregivers should be warned to monitor for any worsening of their condition (including the appearance of new symptoms) and/or the emergence of suicidal ideation/attempts or self-harm in order to promptly seek help if these symptoms occur.
In this case, the situation should be assessed and appropriate changes to the therapeutic regimen should be made, and if necessary, treatment should be discontinued in patients with signs of clinical deterioration (including the appearance of new symptoms) and/or the emergence of suicidal ideation/behavior, especially if these symptoms are severe, occur suddenly and are not part of the existing symptoms.
More details are described in the instructions, or your doctor will inform you.
Overdose of Lamotrin Lamotrigine 100 mg:
Cases of acute overdose (at doses 10–20 times the maximum therapeutic dose), including deaths, have been reported. Symptoms of overdose were: ataxia, nystagmus, impaired consciousness, grand mal seizures, coma. Also, in overdose, widening of the QRS complex (intraventricular conduction delay) has been reported. QRS widening to greater than 100 ms may be associated with more severe toxicity.
The patient must be hospitalized in the intensive care unit for appropriate symptomatic and supportive therapy. Therapy to reduce absorption (activated carbon) should be used if necessary. Additional treatment is prescribed according to clinical indications. There is no experience with the use of hemodialysis to treat overdose. In six volunteers with renal failure, 20% of lamotrigine was eliminated from the body during a 4-hour hemodialysis session.
Side effects:
There are a lot of side effects. Read the instructions or ask your doctor.
Storage:
Store below 25°C in original packaging. Keep out of the reach of children.
Shelf life:
3 years.



Reviews
There are no reviews yet.