Description
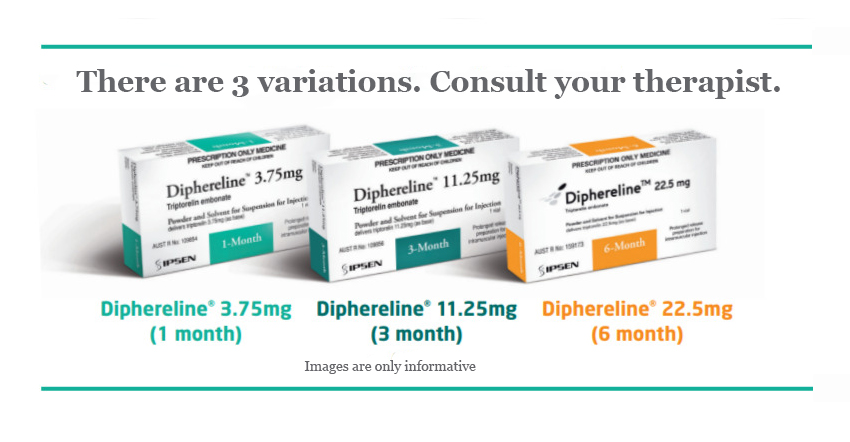
Buy Diphereline Triptorelin 11.25 mg/ml powder with solvent
Description of Diphereline 11,25 mg/ml solution for injection:
Powder and solvent for sustained release injection suspension.
Lyophilisate for the preparation of a suspension for intramuscular administration of a prolonged action of white or white with a creamy tint, dispersible in the attached solvent to form a suspension of white or white with a creamy tint.
The powder is a brittle, slightly yellowish mass.
General characteristics of the reconstituted suspension: homogeneous suspension.
Thinner: colorless, transparent solution.
Ingredients:
1 vial contains triptorelin pamoate corresponding to triptorelin 11.25 mg;
Other components: D, L lactide coglycolide polymer, mannitol (E 421), carmellose sodium, polysorbate 80;
Taking into account the characteristics of the dosage form, each vial contains triptorelin pamoate in an amount corresponding to 15 mg of triptorelin.
Solvent composition:
1 ampoule contains mannitol (E 421), water for injection.
Pharmacological Properties of Diphereline 11,25 mg/ml solution for injection:
Pharmacodynamics:
Triptorelin is a synthetic decapeptide similar to natural GnRH (gonadotropin-releasing hormone).
The results of preclinical and clinical studies have shown that, after initial stimulation, prolonged use of triptorelin suppresses the secretion of gonadotropins with subsequent suppression of testicular and ovarian function.
At the beginning of treatment with Diphereline (11.25 mg), levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the blood may increase, with a corresponding increase in the level (flare) of testosterone in men and estradiol in women. With continued treatment, the levels of LH and FSH decrease, which is accompanied by a decrease in the levels of testosterone and estradiol to post-castration values, which were achieved within about 20 days after injection and persisted until the release of the active substance.
Prostate cancer
An open-label, uncontrolled, multicenter, phase III clinical trial lasting 6 months was conducted in 126 patients to evaluate the effectiveness of subcutaneous Diphereline (11.25 mg) (one injection every 3 months). After 4 weeks, 97.6% of participants achieved a castration testosterone level (<50 ng/dL) (95% confidence interval (CI) 93.2–99.5), which was maintained for 6 months in 96.6% of participants (95% CI 91.6–99.1) (cont. The probability of achieving a castration level during the first month of treatment and maintaining this level at each control time point for 6 months was 96% (95% CI 0.92 – 0.99)
In patients with metastatic castration-resistant prostate cancer, clinical studies have demonstrated the benefit of adding abiraterone acetate as an inhibitor of androgen biosynthesis or enzalutamide as an inhibitor of androgen receptor function to GnRH analogs, such as triptorelin.
Endometriosis
Long-term treatment with the drug Diphereline (11.25 mg) inhibits the secretion of estradiol and thus provides “calm” ectopic endometrium in women.
Precocious puberty
Inhibition of gonadotropic hyperfunction of the pituitary gland in children of both sexes is expressed by inhibition of the secretion of estradiol and testosterone, a decrease in the maximum value of LH and an improvement in growth-age and bone age.
Initial gonadal stimulation may cause minor bleeding, requiring the use of medroxyprogesterone or cyproterone acetate.
Pharmacokinetics:
With intramuscular injection of Diphereline (11.25 mg) in patients (men and women), the peak plasma concentration of triptorelin is observed approximately 3 hours after injection. After a decrease in the concentration phase lasting during the first month, the level of circulating triptorelin in the blood remains constant until the end of the third month after injection.
In a study conducted with the subcutaneous use of this drug in men, peak plasma concentrations of triptorelin were quickly reached after injection (median Tmax = 4.5 h), while the release of triptorelin was constant for 91 days. Three months after subcutaneous administration, residual triptorelin concentrations (Cmin) were 0.063 ng/ml.
Indications for use Diphereline 11,25 mg/ml solution for injection:
Prostate cancerTreatment of locally advanced or metastatic prostate cancer.treatment of high-risk localized or locally advanced prostate cancer in combination with radiotherapy.
The favorable result of treatment is more pronounced and is observed more often if the patient has not previously received any other hormonal therapy.
Genital and extragenital endometriosis (I – IV stages).
Therapy should not be carried out for more than 6 months. Repeated courses of triptorelin or other gonadotropin-releasing hormone (GnRH) analogues are not recommended.
Premature puberty of central genesis in children (in girls under 8 years of age and in boys under 10 years of age).
Contraindications for use Diphereline 11,25 mg/ml solution for injection:
Hypersensitivity to gonadotropin-releasing hormone (GnRH) or any of the excipients. The period of pregnancy or lactation.
Precautions & Warning for use Diphereline 11,25 mg/ml solution for injection:
The use of GnRH agonists can cause a decrease in bone mineral density. Preliminary evidence suggests that in men, the use of a bisphosphonate together with a GnRH agonist may reduce bone mineral density loss. Particular attention should be paid to patients with additional risk factors for osteoporosis (such as alcohol abuse, smoking, long-term treatment with drugs that cause a decrease in bone mineral density, such as anticonvulsants or corticosteroids, a family history of osteoporosis, malnutrition).
Before prescribing the drug Diphereline (11.25) mg, it is necessary to confirm that the patient is not pregnant.
In rare cases, therapy with GnRH agonists can reveal a previously undiagnosed gonadotropic pituitary adenoma. Such patients may experience pituitary apoplexy, characterized by sudden headaches, vomiting, visual disturbances, and ophthalmoplegia.
There is an increased risk of depression (which can be severe) in patients treated with GnRH agonists, in particular triptorelin. With this in mind, patients should be informed and provided with appropriate treatment when symptoms appear. Patients who are depressed need careful monitoring during therapy.
Diphereline® (11.25 mg) contains less than 1 mmol (23 mg) sodium per dose, i.e. it is generally sodium-free.
ATTENTION! It is important that the injection of the extended release medicinal product is carried out in accordance with all instructions given in the instructions for use. Each unsuccessful injection, after which there is more drug in the syringe than prescribed by the instructions, must be recorded.
Side Effects:
General tolerability in men.
Due to the fact that patients with locally advanced or metastatic hormone-dependent prostate cancer are usually elderly and have other diseases that are common in patients in this age group, more than 90% of patients who participated in clinical studies experienced adverse events and were often difficult to assess. Based on data from treatment with other GnRH agonists or after surgical castration, the most frequently observed adverse reactions and those associated with triptorelin therapy were due to the expected pharmacological action. These effects included hot flushes and decreased libido. With the exception of immunoallergic reactions (rare) and reactions at the injection site (< 5%), all adverse events are known to be related to changes in testosterone levels.
The following adverse reactions have been reported and considered at least likely to be related to triptorelin therapy. The occurrence of most of these phenomena is associated with biochemical or surgical castration.
Interaction:
Interaction with other medicinal products and other forms of interaction
When using triptorelin together with drugs that modify the secretion of pituitary gonadotropic hormones, precautions must be taken, and careful monitoring of hormonal levels is recommended.
Since androgen deprivation therapy can prolong the QT interval, the advisability of the simultaneous use of Diphereline with drugs that can prolong the QT interval, and with drugs that can cause torsades de pointes, such as antiarrhythmic classes, must be carefully evaluated. allol, dofetilide, ibutilide), methadone, moxifloxacin, antipsychotics, etc..
Overdose:
In case of overdose, symptomatic treatment is prescribed.
Storage:
The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Shelf life:
2 year.



Reviews
There are no reviews yet.